What happens when you deform a metal?
- Owain Houghton
- Jun 1, 2022
- 3 min read
Updated: Oct 21, 2022
Deforming and shaping metal is an integral part of making jewelry. Bending, rolling, and forming in the absence of heat are referred to as "cold working" an alloy. Understanding how metal behaves when we are shaping it is an integral part of avoiding failure and cracking.
A quick review of the structure of metals
Metals are crystalline. Atoms are arranged in a pattern that repeats in all three dimensions. This can be described by a lattice. Jewelry metals are polycrystalline. This means they are made up of many small crystals. These crystals will have the same structure and composition in a pure metal but are oriented differently. They are known as grains. Because of their different orientation, the grains do not pack perfectly, and the regions that separate them are known as grain boundaries.

An atomic view of deformation
Plastic (irreversible) deformation occurs by layers of atoms sliding over one another. This is known as "slip". A type of crystal defect known as a dislocation is necessary for the slip to occur. These type of defects are specific to each crystal. Strengthening mechanisms work by hindering (resisting) the motion of dislocations, and so a larger force (stress) is needed for the slip to occur.

Analogy to slip – A moving caterpillar
The concept of dislocations and slip is not easy to understand. However, many of you will have seen how a caterpillar moves.
A caterpillar does not attempt to pull itself along by dragging its entire body. It moves by creating a small hump in its body, which then moves along the length of its body. For each passage of a hump from tail to head, the caterpillar moves forward a small amount without the great force needed to drag its whole body along the floor!
Similarly, a crystal doesn't move by the full sliding motion of a layer of atoms. The passage of each dislocation defect allows the crystal to slide a small amount.
Slip leads to a change in shape and orientation of grains in the microstructure, and ultimately a change in shape of the material.
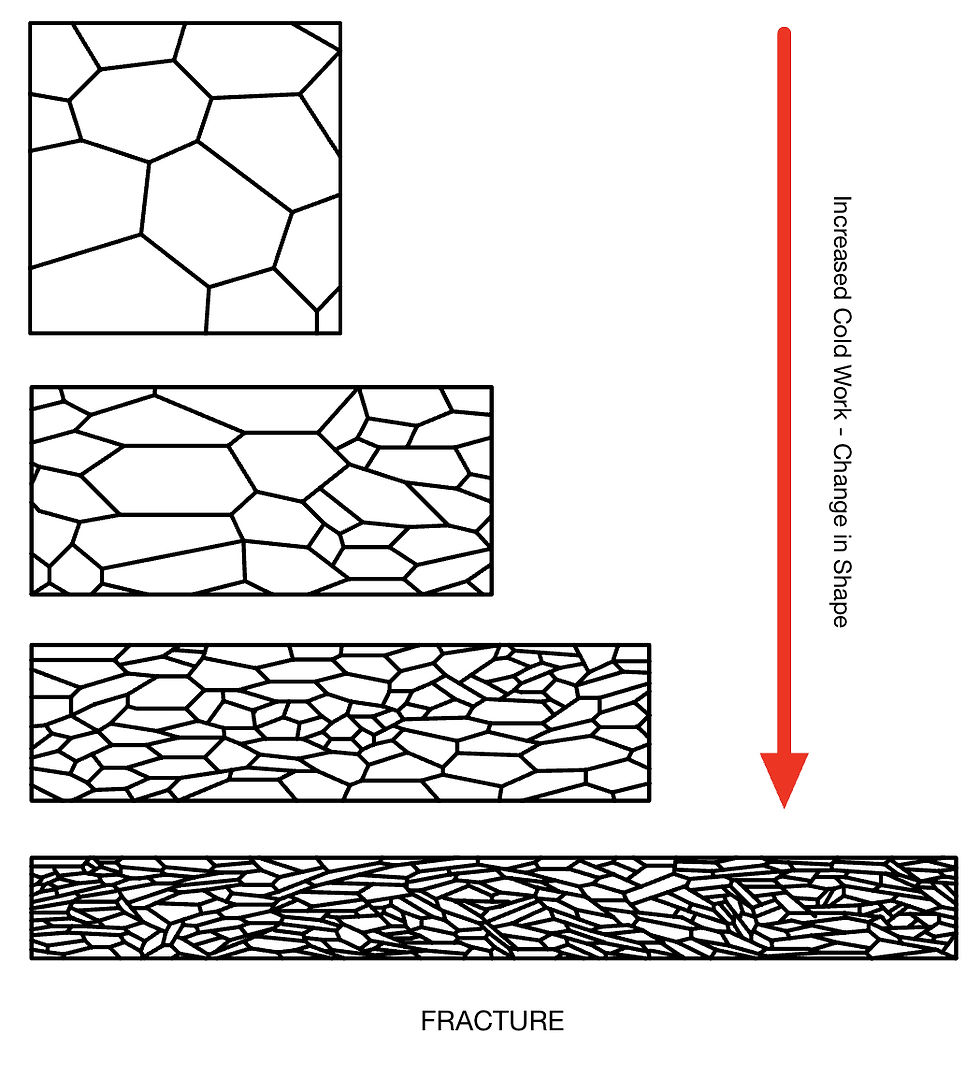
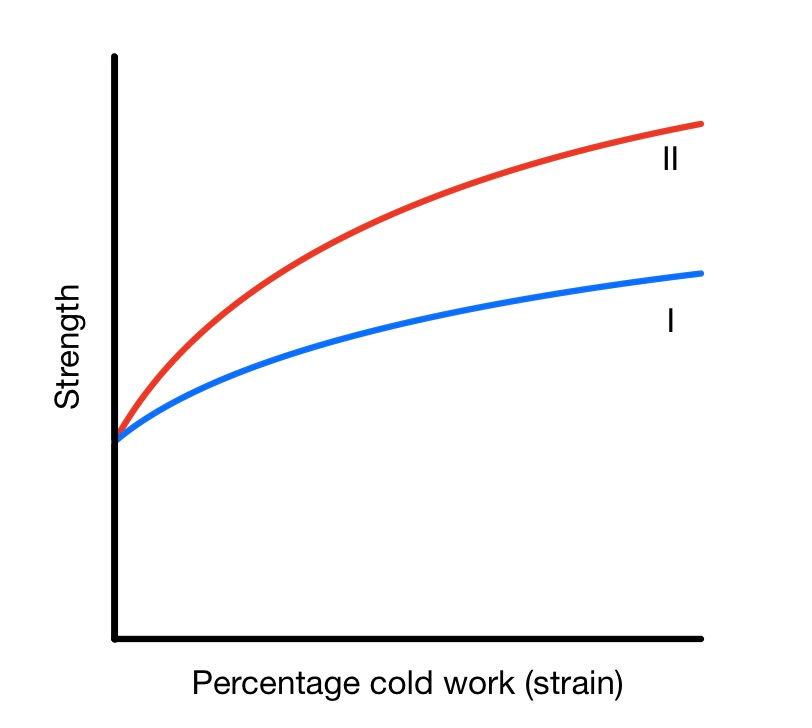
As a material deforms, it gets harder, stronger, and more difficult to deform further. More defects hinder slip further, so a larger force is required. Further ductility is limited because the grains can't deform much more, and the defects generated hinder deformation (they interlock, which stops any further deformation).
The extent of hardening depends on the alloy composition. For example, gold-silver alloys harden slower than gold-copper alloys.
Effect of deformation on the microstructure
In addition to a change in the shape and orientation of grains, slip gives rise to characteristic deformation bands within the grains. Such sliding occurs over several crystal planes in a complex way that gives rise to these bands.

Such deformation is also visible in the overall macrostructure. The microstructure isn't uniform as it appears fibrous. Most cold-working processes result in uneven deformation through the cross-section. For rolling or extrusion, most deformation occurs at the surface, especially if the reduction in thickness for each pass is small.
Effect of deformation on the properties
When we deform a metal, we see several things:
A macroscopic shape change.
A change in grain structure – grains become elongated and reoriented.
An increase in strength – metals become harder due to work hardening.
A reduction in further ductility – Grains are heavily deformed and cannot deform much further. Further working will cause the metal to fracture.
As we deform a metal. there is a reduction in grain size. For practical purposes, “large” will usually mean grains of the order of millimeters or larger, and “small” will refer to grain sizes of the order of tenths or hundredths of a millimeter (1–100 microns).
As we reduce the grain size, then there are more grain boundaries. This means there are more defects in our microstructure. These defects hinder the slip mechanism (layers of atoms sliding over one another – movement of dislocations), so a larger force is required to deform the microstructure. This is a process that occurs during work hardening. To restore ductility, we must anneal the sample to reduce the number of dislocations and restore the grain structure.
After annealing, the grain structure and strength relationship remains true. Hence, annealed metals are softer as the grain size is higher. A fine-grain microstructure is harder and stronger than one with large grains. This is given by the Hall-Petch relationship.

In addition to being stronger and harder, once annealed fine-grained metals are (compared with coarse-grained annealed metals):
More ductile and tougher (less prone to cracking)
Less prone to impurity embrittlement
Less prone to "orange peel" surface after deformation.
ASTM Values
You may also hear of grain sizes referred to in terms of an ASTM numerical value. This is a comparative method of measuring grain size. The higher the number, the smaller the grain size.
Key Points
Deformation of metals occurs by distorting the crystal lattice. Layers of atoms slide over one another by a type of defect known as a dislocation.
Deformation leads to the work hardening of the metal. The metal becomes harder and more brittle due to the work already done.
At a microscopic level, working a metal leads to a change in the shape and orientation of the grain structure.
Reducing the grain size increases a metal's strength (hardness) and toughness (resistance to cracking).
Cover Image courtesy of Chris Ploof.










Comments